Optimal Therapy for Chronic Hepatitis C Genotype 4
Optimal Therapy for Chronic Hepatitis C Genotype 4
Prof. Dr. Imam Waked, Md
Professor of Medicine
Hepatology Department, National Liver Institute, Menoufiya University, Egypt
Abbreviations
Hepatitis C virus genotype 4 (HCV-G4)
pegylated interferon (PEG-IFN)-ribavirin (RBV)
adverse events (AEs)
sofosbuvir (SOF)
simeprevir (SIM)
daclatasvir (DCV)
direct acting antiviral (DAAs)
protease inhibitors (PIs)
boceprevir (BOC)
telaprevir (TVR)
sustained virological response (SVR)
rapid virological response (RVR)
asunaprevir (ASV),
resistance associated variants (RAVs)
ledipasvir (LDV)
ABSTRACT
Optimal therapy for patients with hepatitis C virus (HCV) genotype 4 (HCV-G4) infection is rapidly evolving, and the possibility of a total cure is near. The standard of care has been combination pegylated interferon (PEG-IFN)-ribavirin (RBV), with modest response rates and considerable adverse events (AEs). Since the introduction of sofosbuvir (SOF), ledipasvir (LDV), simeprevir (SIM), daclatasvir (DCV), ombitasvir (OBV) and ritonavir-boosted paritaprevir (PTV/r), the duration of treatment has been significantly reduced and response rates have increased.
The recommended treatment for IFN eligible patients is PEG-IFN/RBV plus SOF, SIM, or DCV. In IFN ineligible patients, treatment options include LDV/SOF for 12 weeks, OBV/PTV/r for 12 weeks, a 24-week course of SOF/RBV, and a 12-week course of the SOF-SIM or SOF-DCV with or without RBV.
The pipeline for patients with chronic HCV is highly active. IFN-free combinations with for 12 weeks or less with close to 100% cure rates will soon become the optimal therapy.
Key points
• Until recently, the combination of pegylated interferon (PEG-IFN) and ribavirin (RBV) was the only available treatment option for HCV-G4 infection.
• Current treatment options include the addition of recently approved direct acting antiviral (DAAs) to PEG-IFN/RBV or a combination of DAAs.
• Newer combinations of DAAs with higher cure rates, a shorter treatment duration, a higher genetic barrier, and minimal adverse events will be available in the near future
Introduction
HCV genotype 4 (HCV-G4) is responsible for approximately 20% of cases of chronic HCV infection worldwide (1, 2), and more than 90% of the cases in Egypt (3) which has the highest HCV prevalence worldwide (4). The number of cases of HCV-G4 has recently increased in southern Europe, particularly in France, Spain and Italy (5-7).
The efficacy of combination pegylated interferon (PEG-IFN) and ribavirin (RBV) therapy in patients with HCV-G4 is better than in those with HCV-G1 but less effective than in those with HCV-G2 and G3 (8). With the introduction of specific HCV-G1 protease inhibitors (PIs) boceprevir (BOC) (9) and telaprevir (TVR) (10) in 2011, HCV-G4 became the “most difficult to treat” genotype. The approval of sofosbuvir (SOF) (11), simeprevir (SIM) (12), daclatasvir (DCV) (13) and the combination of ombitasvir (OBV) and ritonavir boosted-paritaprevir (PTV/r) (13) for the treatment of HCV-G4 led to significant improvement in response to therapy
Interferon-ribavirin therapy
In the past decade, dual therapy with PEG-IFN/RBV for 48 weeks has been the standard of care in patients with HCV-G4. This regimen is still the only available therapy in many parts of the world, but this is rapidly changing.
Real-life data of PEG-RBV therapy: 40-55% sustained virological response
In the PROPHESYS worldwide
real-life cohort of 7,163 treatment-naïve patients with HCV, who were treated with PEG-IFN/RBV, only 41% of HCV-G4 infected patients achieved a sustained virological response (SVR) (14). The Egyptian national program for the treatment of hepatitis C treated approximately 350,000 patients between 2007 and 2014 (15). Although the program excluded difficult to cure patients (those with F4 fibrosis, previous treatment failures, high BMI and age over 60), their published real-life data showed an SVR rate of 45%-55% (16).
Baseline Predictors of response: fibrosis, viral load, IL-28B genotype, insulin resistance and virus subtype
Multiple host and viral factors can help predict SVR rates in HCV-G4 patients treated with PEG-IFN/RBV (17-19). These include baseline fibrosis, where real-life data showed an SVR of 27.5% in patients with bridging fibrosis or cirrhosis on liver biopsy compared to 41.6% in those without (14) and pre-treatment viral load, where patients with high viral load (>800,000 IU/ml) had lower SVR than those with low viremia (31.6% vs. 63.1%).
The major predictor of response to PEG-IFN/RBV is the IL-28B genotype (20, 21). The IL-28B rs12979860 CC genotype is associated with the highest SVR rates (between 66% and 80%) while patients with the TT genotype have SVR rates between 20% and 30% and CT heterozygote patients had SVR rates between 40 and 50%.
HCV-G4 patients with insulin resistance (HOMA-IR scores >2) have lower SVR rates than those with scores <2, which partially
improve when pioglitazone is added to PEG-IFN/RBV (22).
Ethnicity and HCV-G4 subtype also influence treatment response to PEG-IFN/RBV, and Egyptian patients responded better than French/European or African patients infected with HCV-G4 (SVR 54.9%, 40.3% and 32.4%, respectively, p<0.05) (19). The overall response rate was better in patients infected with the HCV-G4 subtype 4a (60% vs. 35% for non-4a subtypes), and the predominance of subtype 4a in Egyptian patients (93% compared to 54% in French patients, and 11.5% in patients from sub-Saharan Africa) may explain this variation in response rates to PEG-IFN/RBV.
Response Guided Therapy (RGT): importance of a rapid virological response
The SVR rate is better in HCV-G4 infected patients who achieve a rapid virological response (RVR- HCV-RNA negative 4 weeks after beginning PEG-IFN/RBV)(23). Several studies have shown that response rates are similar in patients with an RVR treated for 24 or 48 weeks and an international expert panel (24) recommended shortening
therapy to 24 weeks in patients with an RVR without negative baseline predictors of response (high viral load, advanced fibrosis, and insulin resistance).
Future of PEG-IFN/RBV Therapy
The impact of preselecting HCV-G4 patients with multiple positive predictors of response to treatment on the outcome of PEG-IFN/RBV therapy requires further validation. However, as effective DAAs become available there will be no further need for 48 weeks of PEG-IFN/RBV therapy in HCV-G4 patients.
Direct Acting Antivirals: a rapidly changing scene
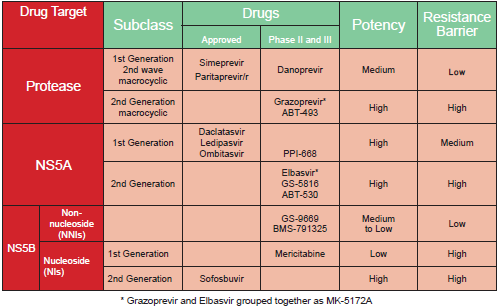
The HCV replication complex has several drug target sites, and current DAAs include NS3/NS4 PIs, NS5A inhibitors, and NS5B polymerase inhibitors (table 1). Although the first generation PIs, BOC and TVR were not effective in HCV-G4, they were precursors to the development of more potent PIs and other classes of DAAs that are effective in HCV-G4 patients (25).
Results of trials with DAAs in patients with HCV-G4: second generation PIs, NS5A and NS5B inhibitors
First generation linear PIs BOC and TVR were not indicated for treatment of HCV-G4 because of lack of efficacy due to a high incidence of resistance.On the other hand
generation macrocyclic PIs [SIM, asunaprevir (ASV), and ritonavir-boosted paritaprevir (PTV/r)] are more effective in HCV-G4 patients.
With SIM, the reduction in plasma HCV-RNA from baseline was greater in patients with HCV-G1 and G4 than in those with HCV-G2 and G5, with no antiviral activity against HCV-G3 (26, 27). When it was given once a day orally for 12 weeks with PEG-IFN/RBV for 24-48 weeks, SIM improved the SVR in HCV-G4 treatment-naïve and experienced patients (28). SVR12 was achieved in 83% of treatment-naïve patients, 86% of prior relapsers, 60% of prior partial responders, and 40% of prior non-responders. Shorter 12-week treatment is currently being evaluated in HCV-G4 treatment-naïve patients without cirrhosis based on early viral kinetics (ClinicalTrials.gov Identifier: NCT01846832).
The addition of the NS5A inhibitor DCV to a 24-week course of PEG-IFN/RBV improved SVR rates in HCV-G4 patients to 67% with a single oral daily dose of 20 mg, and to 100% with a 60 mg dose compared to 50% with standard 48-week PEG-IFN/RBV treatment(28).
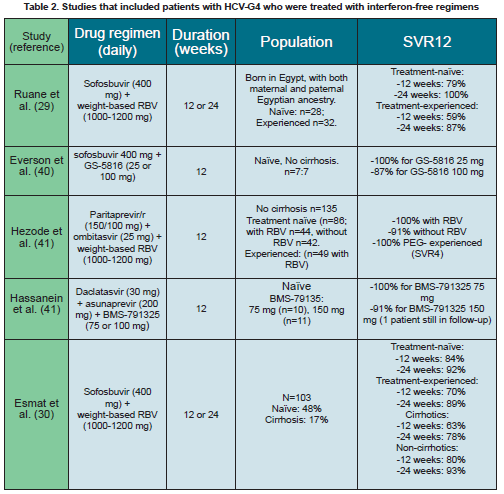
The addition of the NS5B nucleoside inhibitor SOF for 12 weeks to PEG-IFN/RBV therapy resulted in an SVR rate of 96% in treatment-naïve HCV-G4 patients (11). SOF given for 12 weeks in combination with RBV but without IFN to HCV-G4 patients of Egyptian origin in the US resulted in SVR rates of 79% and 59% in treatment-naïve and experienced patients respectively (29) (table 2). Extending treatment for 24 weeks improved the SVR to 100% and 93% respectively. In patients with cirrhosis, the SVR12 in treatment-naïve patients was 91% with 12 weeks of therapy vs.100% with 24 weeks of therapy and 50% vs. 100% in treatment-experienced patients. In a similar trial in Egypt including 103 patients treated with SOF and RBV for 12 or 24 weeks, SVR12 rates were higher with 24 weeks of therapy (90% vs. 77%). Treatment-naïve patients had a better response than experienced patients after 12 weeks (84% vs. 70% respectively) and 24 weeks of therapy (92% vs.
89% respectively). Patients without cirrhosis also responded better than those with cirrhosis after 12 (80% vs. 63%), and 24 weeks of treatment (93% vs. 78%, respectively) (30) (Table 2).
DAA Resistance
HCV replicates at a high rate, and produces approximately ~1012 virions every day. The NS5B RNA dependent RNA polymerase has a high error rate, leading to possible nucleotide substitutions in the HCV genome with every replication cycle. This leads to the natural occurrence of virions with one or more nucleotide substitutions conferring baseline resistance to DAAs in the untreated patient (baseline resistance associated variants, RAVs) (31). With treatment, baseline RAVs are selected, and eventually become the dominant viral population in the patient.
The HCV NS5A inhibitors DCV, ledipasvir (LDV) and OBV have low barriers for resistance. Single mutations at Q30E and Y93N confer high levels of resistance to NS5A inhibitors, and were detected in 13%-15% of HCV-G1 and 4.3% of HCV-G4 treatment-naïve patients (32). Treatment failure in patients who received DCV 20 mg with PEG-IFN/RBV, was associated with the emergence of L28M-L30H and L28M-L30S RAV (28).
SOF has a high genetic barrier for resistance. Three amino acid changes were identified in subjects who received SOF in phase 3 trials: L159F and V321A which do not appear to influence the activity of SOF, and S282T which resulted in a 13.5-fold reduced susceptibility to SOF in one patient (33). The S282T mutant remained fully susceptible to the PI vedroprevir (GS-9451), the NS5A inhibitor LDV, and the NNIs tegobuvir and GS-9669 (34).
Although baseline RAVs exist, and resistance inducing mutations
develop with PIs and NS5A inhibitors, combining DAAs with different targets of activity limits the effects of these mutations. Patients who failed previous treatment with PIs and had a dominant RAV viral population (mainly R155 and/or V36M) responded in a similar manner to the combination of SOF-DCV as those without baseline RAVs (35). In addition, baseline NS5A RAVs present in 140 of 861 treatment-naïve patients (16%) did not prevent SVR with a SOF-LDV combination (36).
All-oral DAA combinations in the very near future
IFN-free DAA combinations with very high cure rates overcome baseline RAVs, and are in the final stages of approval (25) (Table 2).
The combination of SOF-LDV in a single oral daily fixed dose resulted in an SVR in >93% of patients after 8 weeks in HCV-G1 treatment-naïve patients, and >97% after 12 weeks and resulted in SVR rates >93% after 12 weeks in previous non-responders with or without RBV (37, 38). This combination is currently being evaluated in patients with HCV-G4 (ClinicalTrials.gov Identifier: NCT02081079), and when given for 12 weeks to 20 patients with HCV-G4 infection (including 40% treatment experienced and 40% with advanced fibrosis) resulted in complete on-treatment viral suppression and SVR in all except one non-compliant patient, for up to 12 weeks after end of therapy (39).
The combination of SOF with the NS5A inhibitor GS-5816 administered for 12 weeks to154 patients resulted in an overall SVR rate >95%, including 13/14 HCV-G4 patients (40) (Table 2).
An all-oral combination of PTV/r, and OBV, the NS5A inhibitor, for 12-weeks in HCV-G4 patients without cirrhosis resulted in SVR12 rates of 100% with RBV and 91% without RBV in treatment-naïve patients, and 100% SVR4 in experienced patients. Three patients experienced treatment failure due to virological breakthrough or relapse. They had RAVs in the NS3 (D168V) and NS5A (L28V) regions at the time of failure (41) (Table 2). Phylogenetic analysis was performed on a region of the NS5B gene from 132 G4 baseline samples to determine the sub-genotype in this study. Regardless of the subtype, HCV G4-infected patients treated with OBV/PTV/r with RBV achieved an SVR12 of 100%, indicating that this therapy regimen may not require prior specific G4 subtype identification (42).
The all-oral RBV-free combination of DCV, ASV, and the NS5B NNI BMS-791325 in treatment-naïve patients with HCV-G4, was safe, well tolerated and resulted in an SVR12 in 100% of patients, with no virological failures (41) (Table 2).
Although larger trials are needed existing results show a nearly total cure for HCV-G4 with IFN-free, DAA combination therapy. Real life data from the large number of patients with advanced fibrosis (F3-F4) being treated in Egypt will show the impact of DAAs on the cure of HCV-G4.
Predictors of response in interferon-free regimens: still useful?
Potent second-generation DAA combinations, with SVR rates of more than 90% will probably eliminate the pertinence of predictive factors of response to PEG-IFN/RBV therapy such as IL-28B status, viral load, race, metabolic syndrome, obesity and age. Prior failure of response to PEG-IFN/RBV will not prevent response, and even prior non-responders will do almost as well as treatment-naïve patients with cure rates ~90%. Moreover, since almost all patients become HCV-RNA negative within 4 weeks, rapid and early viral response will no longer be pertinent. Advanced fibrosis and cirrhosis may continue to be determining factors, and will probably require more potent combinations or longer treatment durations.
Next generation DAAs are therefore easy to use, for a shorter duration, and provide an almost complete cure for HCV-G4.
Patients with decompensated cirrhosis: a new hope?
Although it is not known whether treating HCV patients with decompensated cirrhosis will prevent further decompensation and reverse progression, certain results have been published. Fifty patients with decompensated liver disease and HCV genotypes 1-4 were randomized in a phase II trial to receive SOF and RBV for 48 weeks (n=25) or 24 weeks followed by cross-over to the same treatment doses and duration (43). Interim results at week 24 showed that with treatment, HCV RNA was undetectable in 100% of Child-Pugh class A patients at week 4, and 93% of Child-Pugh class B patients at week 24. All treated patients who initially had ascites and encephalopathy experienced complete regression, while in the observation arm, encephalopathy increased, and ascites regressed in only 2/9 patients who had ascites initially.
In another multicenter study, 108 patients with decompensated cirrhosis [Child-Pugh class B (score 7-9) and C (score 10-12)] related to HCV genotypes 1 and 4, were randomized to receive SOF/LDV and RBV for 12 or 24 weeks. This regimen was generally safe and well-tolerated. The SVR12 rates were similar for patients with Child-Pugh class B (87% and 89% for 12 and 24 weeks, respectively) and class C (86% and 90% for 12 and 24 weeks, respectively). Significant decrease in bilirubin levels and increase in albumin levels were observed from baseline to week 4 post-treatment. Most patients had an improvement in their Child-Pugh and MELD scores (44).
Whether these preliminary observations will be confirmed in large numbers of patients and trials, and whether patients will improve enough to be taken off the transplant waiting list still remains to be seen
The cost-effectiveness of PEG-IFN/RBV therapy for HCV-G4 has not been extensively studied especially in countries with high prevalence's. A Markov simulation model was used to analyze the cost-effectiveness of PEG-IFN/RBV in Egyptian patients with chronic HCV at different stages of fibrosis until affordable and effective DAAs become available, which the authors presumed to be in 2016 (45). Treatment with PEG-IFN/RBV in Egypt at a reduced cost of $2,000 and response rate of 65% for patients with fibrosis stages F0-F2 and 40%
for F3-F4, was found to be cost-effective for all stages of fibrosis, and the most cost effective in patients with F4. They assumed that the cost of SOF plus PEG-IFN/RBV for 12 weeks would be double the cost of treatment with PEG-IFN/RBV, and yet this option was also cost effective for all stages of fibrosis. However, the negotiated price for SOF obtained by the Egyptian government (46) makes SOF-PEG-INF/RBV triple therapy 40% of the hypothetical price (at $1,500 for a 12 week therapy), and thus more cost effective.
Guidelines: a rapidly evolving field
Medical society and national guidelines will soon be updated to include the approval of SOF, SIM and DCV for the treatment of patients with HCV-G4. However, recommendations will soon be outdated because of the unprecedented rate of development and approval of effective new medications. Guidelines that are less than a year old are now being modified, or have become invalid.
PEG-IFN/RBV based guidelines
Early in 2014 the World Gastroenterology Organization (WGO) considered drafting treatment regimens that were tailored to the resources of different countries (47). These guidelines suggested deferring treatment for patients with minimal-mild disease (F0-F1) of any HCV genotype in regions with limited resources, and reassessing fibrosis after 5-10 years. They recommended that patients in resource-limited regions receive the combination of PEG-IFN/RBV even after 2014, and that an all-oral approach being offered to patients in resource-rich regions and to those who do not respond or relapse following PEG-IFN/RBV in resource-limited regions.
The World Health Organization (WHO) issued its first guidelines for the management of patients infected with HCV in January 2014 (47). They still recommended dual PEG-IFN/RBV therapy, and suggested adopting response guided therapy when deciding on the treatment duration. The panel also recommended adding SOF to PEG-IFN/RBV depending on availability.
These recommendations were based on the initial pricing in the US and Europe for SOF ($84,000 for 12-week supply) and SIM ($60,000 for 12-week supply), which are too expensive in almost all other countries. At the price of $900 for 12 weeks of SOF negotiated between the Egyptian government and Gilead Sciences (46), and the same price agreed to in India and offered to other developing countries (48), these recommendations are now outdated and invalid. Dual
therapy with PEG-IFN/RBV, which is less effective, associated with significantly more AEs, and requires more administrative management, has now become more expensive in resource-limited settings and developing countries.
DAA based Guidelines
On the other hand, the American Association for the Study of Liver Disease (AASLD) and the Infectious Diseases Society of America (IDSA) guidelines (49) no longer recommend PEG-IFN/RBV therapy in patients with HCV-G4 infection. Updated guidelines recommend three treatment options with similar efficacy to treatment-naïve patients with HCV-G4, LDV/SOF for 12 weeks, OBV/PTV/r-RBV for 12 weeks or SOF-RBV for 24 weeks. They suggested the use of SOF-PEG-IFN/RBV for 12 weeks as an acceptable alternative in those patients. They also stated that the previous four options have similar efficacy in patients with partial or no response to prior PEG-IFN/RBV therapy.
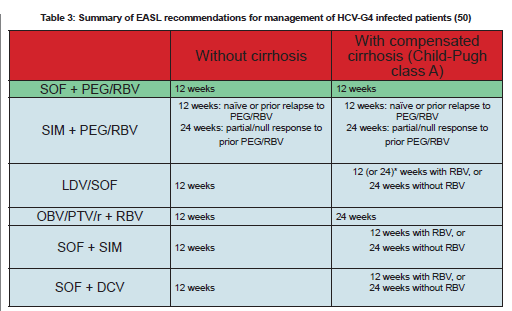
The European Association for the Study of the Liver (EASL) also published HCV treatment guidelines in March 2015 (50). The guidelines include 6 recommended treatment options for patients with HCV-G4 infection. None of these options include dual PEG-IFN/RBV or SOF-RBV therapy (Table 3).
By the time this review has been printed PEG-IFN/RBV for 48 weeks will no longer be prescribed or used in patients with HCV-G4 infection, even in resource-limited countries.
The number and efficacy of
treatment options for HCV and in particular HCV-G4 have increased with shorter treatment and minimal side effects. Whether predictors of response can be identified to select patients for shorter therapy still must be determined. Also the shortest treatment duration must also be determined. At present 8-weeks of SOF-LDV appears to be the shortest effective regimen in treatment-naïve patients. Six-week and four-week regimens are being evaluated in clinical trials for HCV-G1-3 (ClinicalTrials.gov Identifiers: NCT01805882, NCT02133131, NCT02175966) and will definitely influence treatment duration in patients with HCV-G4. Hopefully future combinations or treatments will shorten treatment even further.
Optimal management of HCV-G4 is evolving. Combination DAA therapies is now close to 100% effective, with short duration, oral administration in a single inexpensive daily dose, with minimal AEs, suitable for all patients including those with decompensated cirrhosis and post-liver transplantation, and without resistance. Expert management and monitoring of patients without advanced liver disease will probably not be needed in
the future, and patients will probably be managed by general gastroenterologists, internists and even general practitioners rather than hepatologists. Updated guidelines should adapt to the changing scene and include new therapies until a complete cure has been achieved


 info@utopiapharma.com
info@utopiapharma.com
 Plot No. (2) Industrial Zone (A7) - formerly Zizinia - Cairo - Ismailia Road - 10th of Ramadan - Sharkia
Plot No. (2) Industrial Zone (A7) - formerly Zizinia - Cairo - Ismailia Road - 10th of Ramadan - Sharkia