Early colon cancer: is it now considered a curable disease?
Early colon cancer: is it now considered a curable disease?
Dr.Tamer El Nahas, MD
Professor of Clinical Oncology, Cairo University ESMO & ASCO Member
Introduction
Colorectal cancer (CRC) is the third most common tumour in men and the second in women, accounting for 10%of all tumour types worldwide. Incidence is higher in males (ratio: 1.4) and for both genders there is a 10-fold difference in incidence between several regions. With 608 000 deaths estimated each year (8% of all cancer deaths), CRC is the fourth most common cancer-related cause of death in the world. Mortality has declined progressively in many Western countries: this can be attributed to cancer screening programmes, removal of adenomas, early detection of cancerous lesions and availability of more effective therapies, chiefly for early stage disease. Mortality rates for CRC in the European Union (EU) vary between 15 and 20 of 100 000 males and between 9 and 14 of 100 000 females and have decreased in bothWestern and Northern Countries, particularly in females.
The risk of developing colon cancer depends on factors which can be classified into lifestyle or behavioural factors (such as smoking, high red meat consumption, obesity, physical inactivity) and genetically determinant factors. According to international guidelines, screening tests are stratified according to the personal risk of disease. Age is considered the major unchangeable risk factor for sporadic colon cancer: nearly 70% of patients with colon cancer are over 65 years of age, and this disease is rare before 40 years
Methods of screening
The aim of screening is to detect a precancer condition in a healthy population, as well as very earlystage malignancies which can be treated with a clearly curative intention. Only the faecal occult blood test (FOBT) for men and women aged 50–74 (or 70) years has been recommended to date. In average-risk populations, the guaiac (g) FOBT reduced mortality from CRC by 15% in different age
groups. The benefit from annual screening appears to be greater than for biennial screening and the test interval should not exceed 2 years.
Symptoms
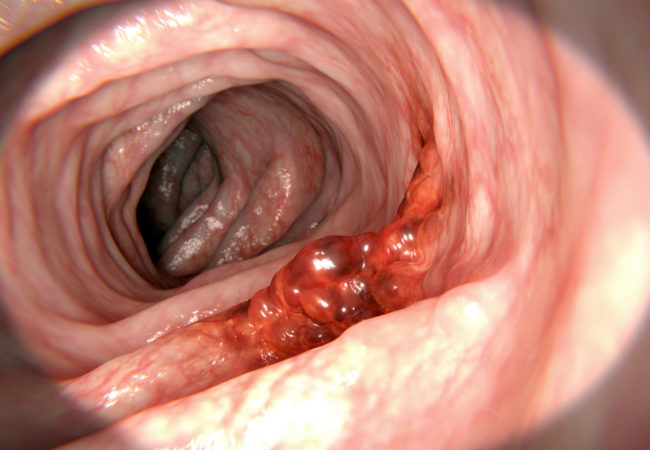
Colon cancer arises from the mucosa of the bowel, generally growing towards the lumen and/or spreading to adjacent organs. Symptoms are associated with relatively large tumours and/or advanced disease stages, and are generally not specific for colon cancer. Change in bowel habits, general or localized abdominal pain, weight loss without other specific causes,
weakness, iron deficiency and anaemia are the most common symptoms, and depends on the location and stage of the primary tumour; they are associated with worse prognosis and their number (but not their duration) is inversely related to survival.
Diagnosis
Endoscopy is the main procedure for diagnosis and can be carried out by either sigmoidoscopy (as >35% of tumours are located in the rectosigmoid) or (preferably) a total colonoscopy.
The advantages of endoscopy are many, e.g. determination of the exact localisation and biopsy of the lesion, detection of (further) synchronous precancerous or cancerous lesions and removal of polyps. Before surgery, if a complete colonoscopy cannot be carried out for whatever reason, the rest of the colon should be visualised by combining limited left-sided colonoscopy with barium enema in order to study the proximal
colon. Virtual colonoscopy or CT colonography are not yet
standard investigations, but are valuable instruments to identify with precision the location of the tumour or to detect synchronous lesions or polyps, and they are potentially helpful for patients eligible for laparoscopic resection. In any case, if not carried out before, a complete colonoscopy should be carried out within 3–6 months after surgery.
The goal of surgery is a wide resection of the involved segment
of bowel together with the removal of its lymphatic drainage.
The extent of the colonic resection is determined by the blood supply and distribution of regional lymph nodes. The resection
should include a segment of colon of at least 5 cm on either side
of the tumour, although wider margins are often included
because of obligatory ligation of the arterial blood supply.
To clearly define stage II versus III and to identify and
eradicate potential lymph node metastases, at least 12 lymph
nodes must be resected.
Treatment options by stage
Stage 0 (Tis N0 M0)
Treatment options are:
(i) Local excision or simple polypectomy.
(ii) Segmentary en-bloc resection for larger lesions not amenable
to local excision.
Stage I (T12- N0 M0)
Wide surgical resection and anastomosis. No adjuvant
chemotherapy.
Stage II A, B, C (T3 N0 M0, T4 a-b N0 M0)
Wide surgical resection and anastomosis. Following surgery, adjuvant therapy should not be routinely recommended for unselected patients. In high-risk patients who present at least one of the previously mentioned clinical high-risk features, adjuvant therapy could be considered in clinical practice.
Stage III (any T, N1-N2, M0)
Wide surgical resection and anastomosis. Following surgery, the standard treatment is a doublet schedule with oxaliplatin and a fluoropyrimidine. Although all three combination regimens are superior to 5-FU/FA alone, FOLFOX4 or XELOX should be preferred to FLOX. When oxaliplatin is contraindicated, monotherapy with infusional or oral fluoropyrimidines should be
preferred to 5-FU/LV.
Adjuvant chemotherapy options
The benefit of combinations with oxaliplatin has been
demonstrated in three landmark trials. In the MOSAIC study, the addition of oxaliplatin to 5-FU/LV (FOLFOX schema),
demonstrated a significantly increased disease-free survival
(DFS) at 3 years, with a reduction in the risk of recurrence of
23% compared with the control arm (LV5FU2).The update at
the 6-year follow-up confirmed the benefit in DFS of adjuvant
treatment with FOLFOX4, and an advantage was also observed
in overall survival (OS), but for stage III patients only. Negative trials are related to irinotecan in combination with
5-FU (bolus or infusional). The CALGB-89803 trial compared 5-FU/LV + irinotecan (IFL) with the Roswell Park
scheme in more than 1200 patients. The trial was prematurely
closed because of an elevated rate of mortality in the IFL group
with respect to the FL regimen (2.2% versus 0.8%). Efficacy
results indicated no improvement in terms of either OS or event-free survival for IFL, when compared with FL. There is currently no role for targeted agents associated with chemotherapy in the adjuvant setting for colon cancer. All trials evaluating bevacizumab, NSABP C-08, AVANT or cetuximab, NCCTG NO147 and PETACC-8 are negative, probably due to different biological characteristics in early when compared with advanced disease.
personalised medicine
In this disease setting, more research is needed to identify
molecular markers which could lead to advances in
personalised medicine


 info@utopiapharma.com
info@utopiapharma.com
 Plot No. (2) Industrial Zone (A7) - formerly Zizinia - Cairo - Ismailia Road - 10th of Ramadan - Sharkia
Plot No. (2) Industrial Zone (A7) - formerly Zizinia - Cairo - Ismailia Road - 10th of Ramadan - Sharkia